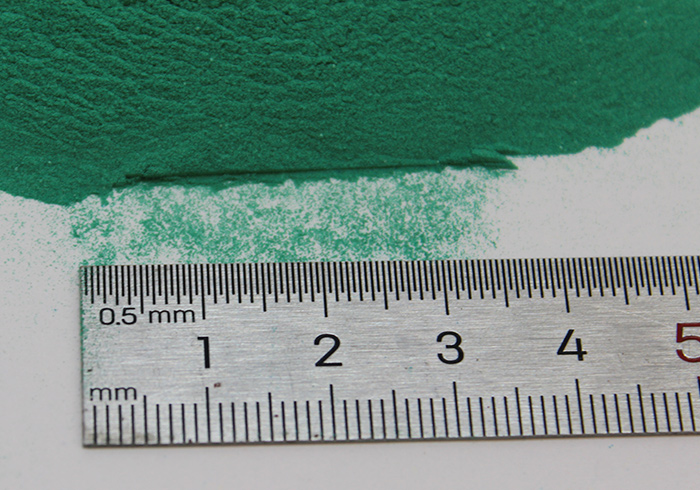
Basic copper carbonate
Product Introduction:
Product Introduction




Product features
Physical and chemical properties:
Green fine amorphous powder, insoluble in water and alcohol, soluble in acid, ammonia, and potassium cyanide, stable at room temperature and pressure. Insoluble in cold water and alcohol, decomposes in hot water, and dissolves in acid to form corresponding copper salts. Dissolve in cyanide, ammonia solution, ammonium salt, and alkali metal carbonate solution to form copper complexes. When boiling in an alkali metal carbonate solution, brown copper oxide forms. Heat to 200 ℃ and decompose into black copper oxide, water (small water droplets formed by condensation when cooled), and carbon dioxide. Unstable in hydrogen sulfide gas. The solubility in water is 0.0008%. Copper carbonate has dustiness and should be avoided from contact with skin, eyes, and inhalation.
The basic copper carbonate with a composition of 2:1 is a sky blue powdery crystal. If left in the air for a long time, it absorbs moisture and releases some carbon dioxide, gradually becoming a 1:1 basic copper carbonate.
Main use:
① Used in organic catalysts, fireworks manufacturing, and pigments;
② Used in agriculture as a preventive agent for plant smut, insecticide, and detoxifying agent for phosphorus toxicity, seed fungicide, mixed with asphalt to prevent livestock and wild rats from gnawing on tree seedlings;
③ Used as a de alkaline agent and raw material for producing copper compounds during crude oil storage.
④ As a feed additive, ruminant animals such as cows have a higher absorption and utilization rate of copper carbonate than other inorganic copper. However, due to its high cost, its application has been limited.
⑤ Used for electroplating, anti-corrosion, and analytical reagents, for preparing oxidation solutions for pre plated silver copper alloys, copper tin alloys, and steel.